
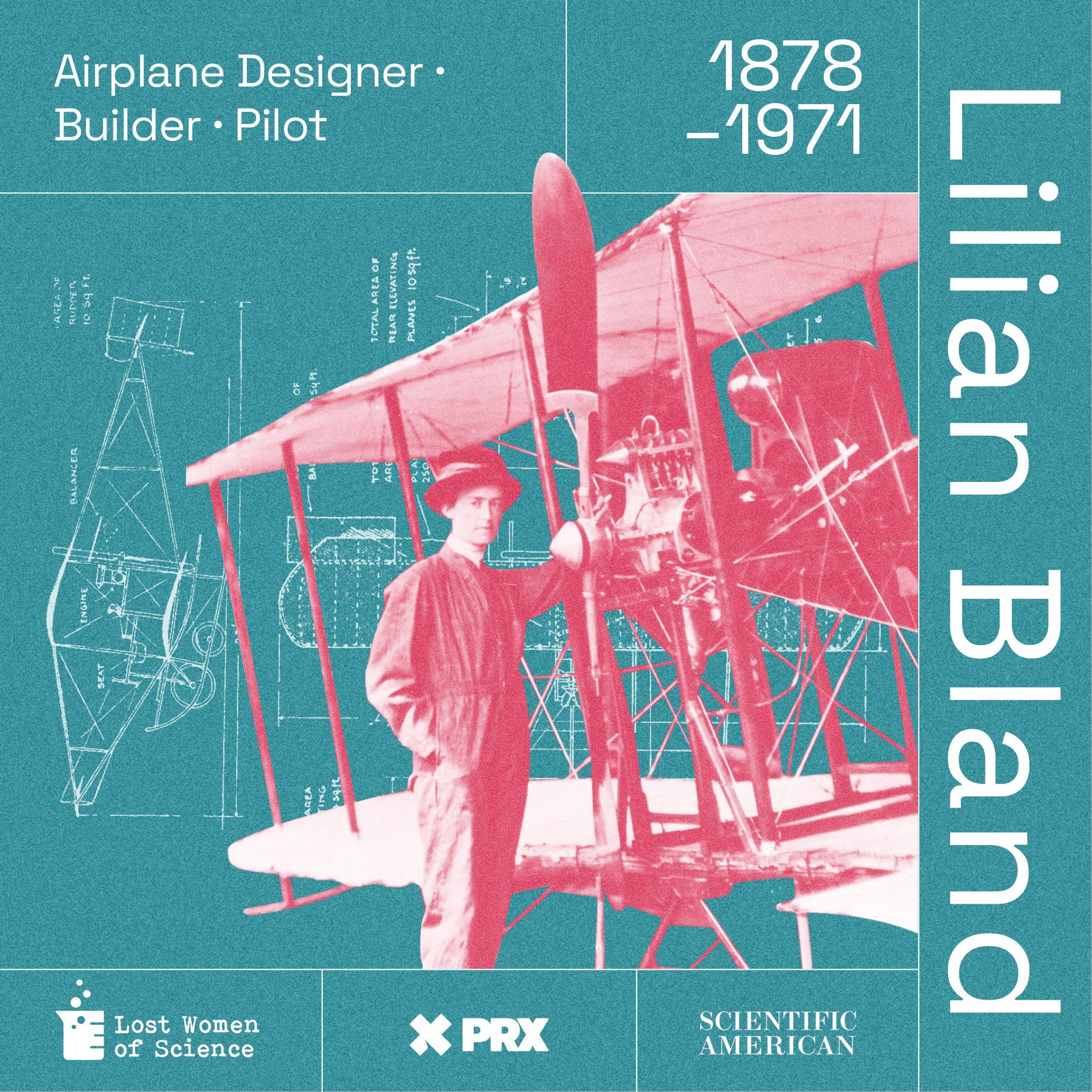
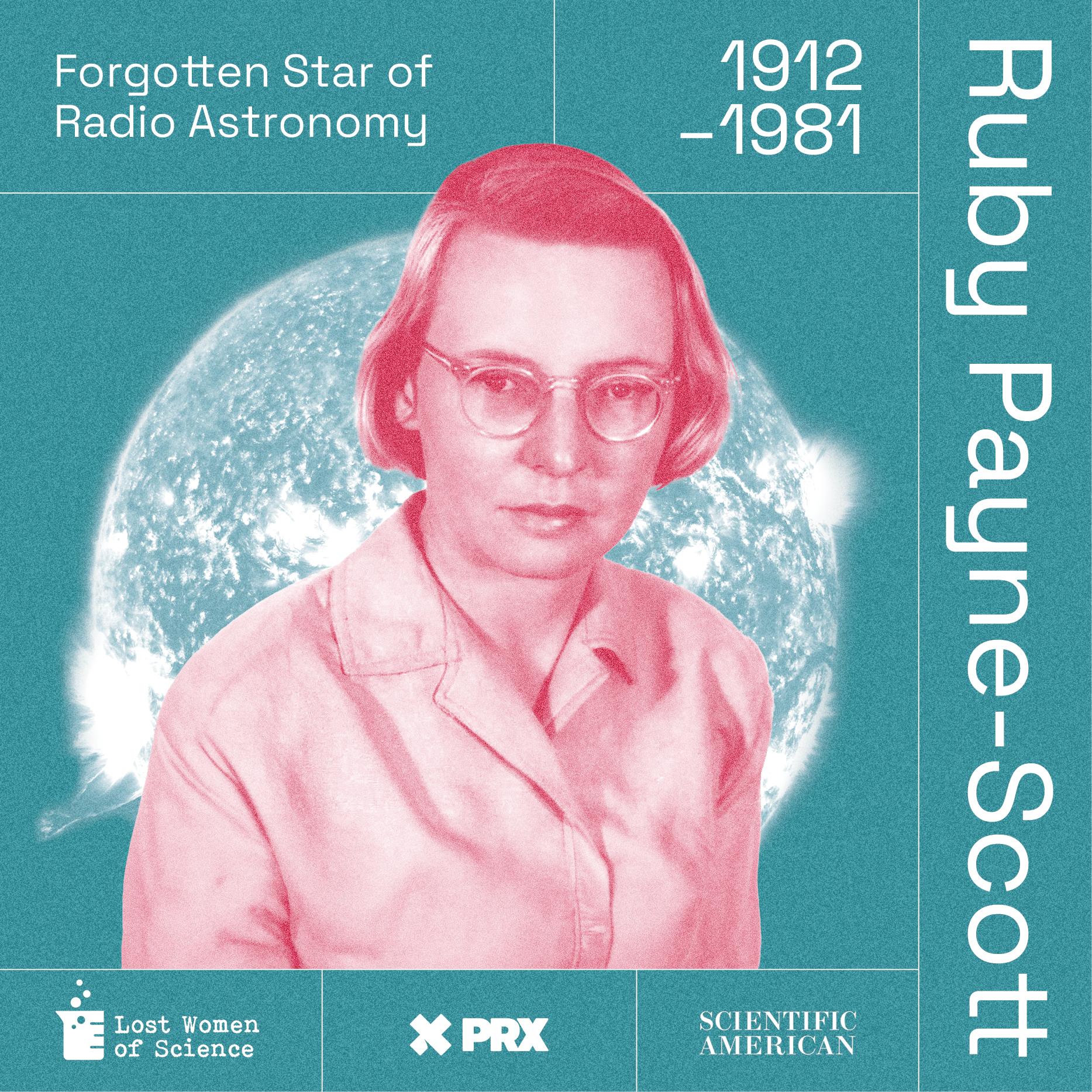
Lost Women of Science
Lost Women of ScienceFor every Marie Curie or Rosalind Franklin whose story has been told, hundreds of female scientists remain unknown to the public at large. In this series, we illuminate the lives and work of a diverse array of groundbreaking scientists who, because of time, place and gender, have gone largely unrecognized. Each season we focus on a different scientist, putting her narrative into context, explaining not just the science but also the social and historical conditions in which she lived and worked. We also bring these stories to the present, painting a full picture of how her work endures.
For every Marie Curie or Rosalind Franklin whose story has been told, hundreds of female scientists remain unknown to the public at large. In this series, we illuminate the lives and work of a diverse array of groundbreaking scientists who, because of time, place and gender, have gone largely unrecognized. Each season we focus on a different scientist, putting her narrative into context, explaining not just the science but also the social and historical conditions in which she lived and worked. We also bring these stories to the present, painting a full picture of how her work endures.














































































The Devil in the Details - Chapter One

In this first chapter of a new five-part season we meet Dr. Frances Oldham Kelsey, a physician and pharmacologist who joined the U.S. Food and Drug Administration as a medical reviewer in 1960. Before the year is out, Dr. Kelsey finds herself standing up to big pharma.
It’s September 1960 and a thick New Drug Application lands on Dr. Kelsey’s desk. The drug has already been on the market in Europe for three years and Dr. Kelsey’s supervisors expect her to rubber stamp the application. The drug is called Kevadon. Active ingredient: thalidomide. And to Frances Kelsey’s keen eye, something looks off.
Learn about your ad choices: dovetail.prx.org/ad-choices